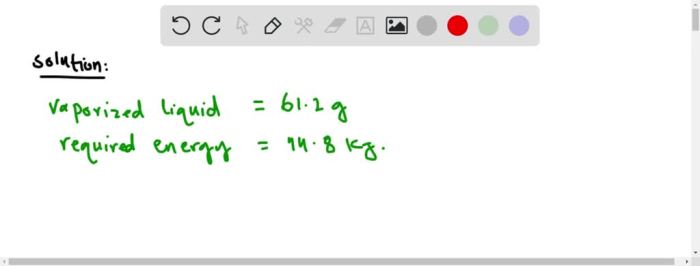
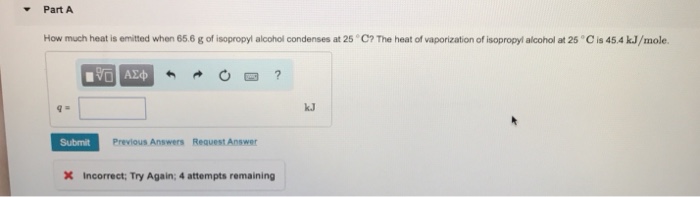
Heat of vaporization isopropyl alcohol, a crucial thermodynamic property, plays a pivotal role in understanding the behavior of liquids and has significant applications in various industries. This article delves into the concept, measurement, and practical implications of the heat of vaporization of isopropyl alcohol.
The heat of vaporization of isopropyl alcohol, numerically expressed in units of kilojoules per mole, represents the amount of energy required to convert one mole of liquid isopropyl alcohol into its vapor phase at a constant temperature and pressure. Several factors, including temperature, pressure, and molecular structure, influence the heat of vaporization of isopropyl alcohol.
Heat of Vaporization
Heat of vaporization refers to the amount of energy required to convert a liquid into a vapor at a constant temperature. It is an important property of liquids that influences their behavior and has significant implications in various scientific and industrial applications.
Heat of Vaporization of Isopropyl Alcohol
Isopropyl alcohol, also known as rubbing alcohol, has a heat of vaporization of 427 kJ/mol. This value represents the energy required to convert one mole of liquid isopropyl alcohol into vapor at its boiling point (82.6 °C).
Factors Influencing Heat of Vaporization
The heat of vaporization of isopropyl alcohol is influenced by several factors, including:
- Molecular weight: Heavier molecules generally have higher heats of vaporization.
- Intermolecular forces: Stronger intermolecular forces, such as hydrogen bonding, require more energy to overcome during vaporization.
- Temperature: The heat of vaporization decreases slightly with increasing temperature.
Applications of Heat of Vaporization of Isopropyl Alcohol
The heat of vaporization of isopropyl alcohol is utilized in various industrial applications, including:
- Disinfection: Isopropyl alcohol is commonly used as a disinfectant due to its high volatility and ability to kill microorganisms.
- Solvent: Isopropyl alcohol is a versatile solvent used in cleaning, degreasing, and extracting various substances.
- Deicing: The high heat of vaporization of isopropyl alcohol makes it effective in deicing applications, such as removing ice from windshields and aircraft.
Safety Considerations
Isopropyl alcohol is a flammable liquid and should be handled with care. Precautions include:
- Avoid contact with eyes and skin.
- Use in well-ventilated areas.
- Store in a cool, dry place away from ignition sources.
- Dispose of properly according to local regulations.
Comparison with Other Solvents, Heat of vaporization isopropyl alcohol
| Solvent | Heat of Vaporization (kJ/mol) |
|---|---|
| Isopropyl alcohol | 427 |
| Ethanol | 387 |
| Acetone | 523 |
| Water | 2,256 |
Isopropyl alcohol has a higher heat of vaporization than ethanol but lower than acetone. This difference in heat of vaporization influences their volatility and evaporation rates, which are important considerations in solvent selection.
FAQ Guide: Heat Of Vaporization Isopropyl Alcohol
What is the heat of vaporization?
The heat of vaporization is the amount of energy required to convert one mole of a liquid into its vapor phase at a constant temperature and pressure.
What is the heat of vaporization of isopropyl alcohol?
The heat of vaporization of isopropyl alcohol is 42.1 kJ/mol.
What are the applications of the heat of vaporization of isopropyl alcohol?
The heat of vaporization of isopropyl alcohol is used in various applications, including solvent extraction, drying, and cooling.