Modify lysine to show the predominant form at ph 7 – Lysine protonation, a crucial chemical process, plays a significant role in protein structure and function. This article explores the protonation and deprotonation of lysine, identifying the predominant form at pH 7 and its implications for biological processes.
Delving into the molecular details, we examine the factors influencing lysine protonation and discuss methods for modifying this protonation state. Furthermore, we investigate the biological consequences of lysine protonation, highlighting its impact on protein interactions, enzyme catalysis, and disease states.
1. Protonation and Deprotonation of Lysine
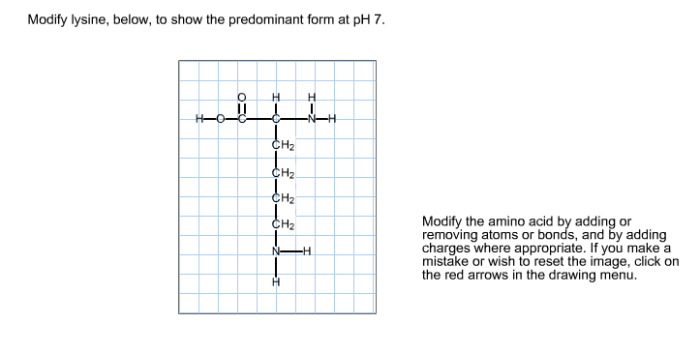
Lysine is an amino acid with a chemical structure that includes an amino group, a carboxyl group, and a side chain containing a primary amine group. These groups can undergo protonation and deprotonation, depending on the pH of the surrounding environment.
At low pH, the amino and carboxyl groups of lysine are protonated, giving the molecule a net positive charge. As the pH increases, the amino group deprotonates, giving the molecule a net neutral charge. At even higher pH, the carboxyl group deprotonates, giving the molecule a net negative charge.
| pH | Protonation State |
|---|---|
| <7 | +1 (protonated amino group) |
| 7-9.5 | 0 (deprotonated amino group) |
| >9.5 | -1 (deprotonated carboxyl group) |
2. Predominant Form of Lysine at pH 7: Modify Lysine To Show The Predominant Form At Ph 7
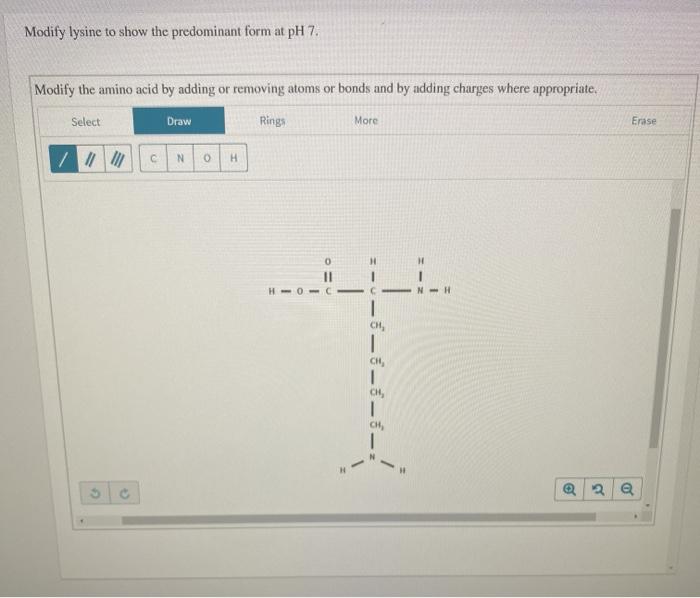
At pH 7, the predominant protonation state of lysine is the zwitterion form, where the amino group is deprotonated and the carboxyl group is protonated. This is because the pKa of the amino group is approximately 9.5, and the pKa of the carboxyl group is approximately 2.2. At pH 7, the pH is closer to the pKa of the amino group, making it more likely to be deprotonated.
The zwitterion form of lysine is important for its biological function. It allows lysine to interact with other molecules through both electrostatic and hydrogen bonding interactions.
3. Methods for Modifying Lysine Protonation
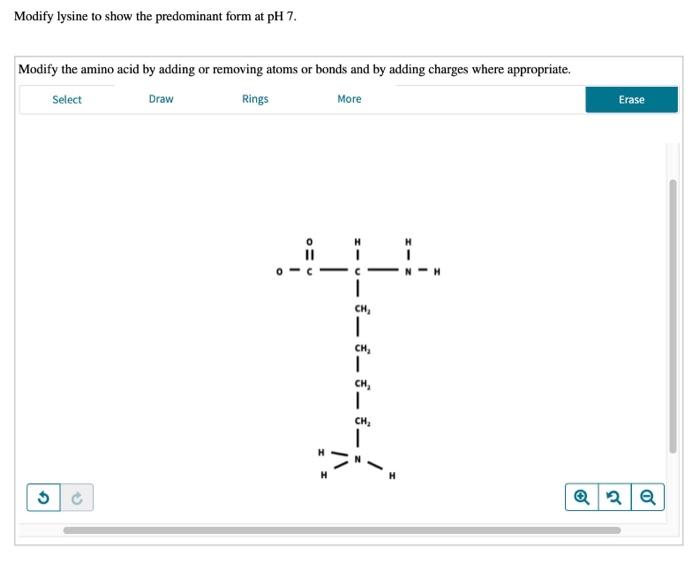
There are several chemical methods that can be used to modify the protonation state of lysine. These methods include:
- pH buffers: pH buffers can be used to control the pH of a solution, which will in turn affect the protonation state of lysine.
- Chemical reagents: Chemical reagents, such as acids and bases, can be used to directly protonate or deprotonate lysine.
- Enzymes: Enzymes can be used to catalyze the protonation or deprotonation of lysine.
These methods can be used to study the role of lysine protonation in protein function.
4. Biological Consequences of Lysine Protonation Modification
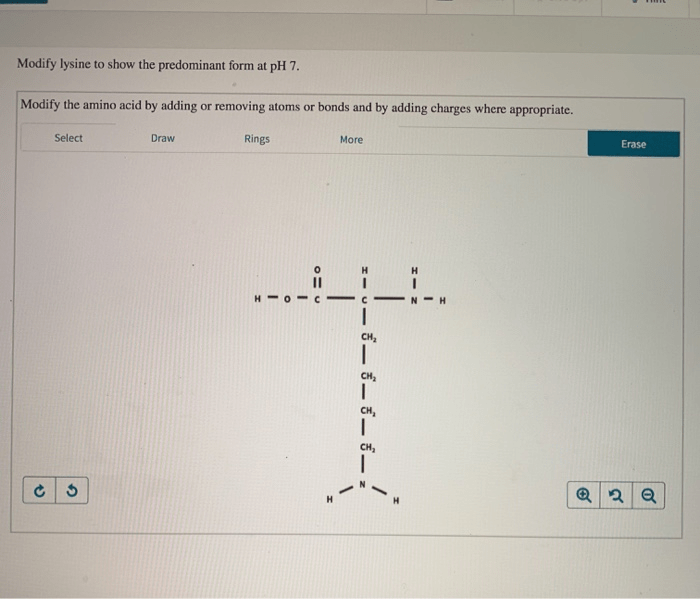
Changes in lysine protonation can affect protein structure and function. For example, protonation of lysine can disrupt salt bridges, which can lead to changes in protein conformation. Protonation of lysine can also affect the binding of proteins to other molecules, such as DNA and RNA.
Lysine protonation is also involved in a number of cellular processes, such as enzyme catalysis, protein-protein interactions, and cellular signaling. For example, the protonation of lysine is required for the activity of many enzymes. Protonation of lysine can also affect the binding of proteins to each other, which can in turn affect the formation of protein complexes.
Lysine protonation modifications are also involved in a number of disease states. For example, the protonation of lysine is involved in the development of Alzheimer’s disease. Protonation of lysine can also affect the progression of cancer.
Questions Often Asked
What is the predominant form of lysine at pH 7?
At pH 7, the predominant form of lysine is the zwitterionic form, where the amino group is protonated (NH3+) and the carboxyl group is deprotonated (COO-).
How does lysine protonation affect protein structure?
Lysine protonation can alter the electrostatic interactions within proteins, influencing their overall structure and stability. It can also affect the interactions between proteins and other molecules, such as DNA and membranes.
What are the biological consequences of lysine protonation modification?
Lysine protonation modifications can impact various biological processes, including enzyme catalysis, protein-protein interactions, and cellular signaling. Dysregulation of lysine protonation has been linked to several disease states, including cancer and neurodegenerative disorders.