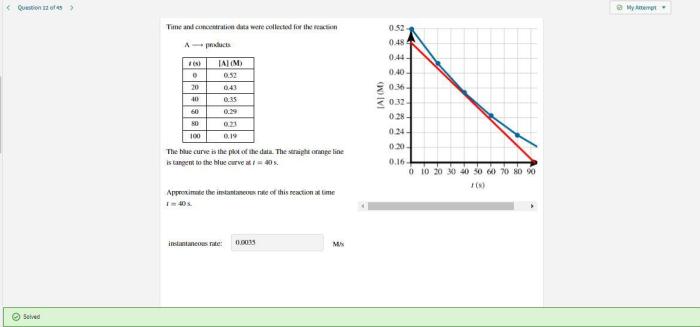
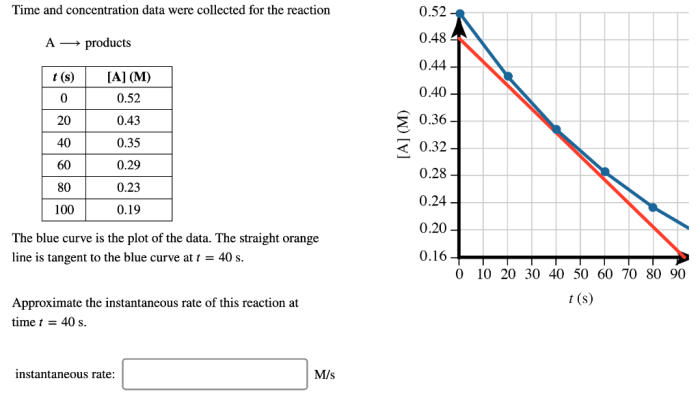
Time and concentration data were collected for the reaction – Time and concentration data are crucial for understanding the kinetics and mechanisms of chemical reactions. This article presents a comprehensive overview of the methods used to collect time and concentration data, the techniques for data analysis and interpretation, the factors that influence reaction time and concentration, and the practical applications of the collected data.
Time and concentration data are collected using various methods, including spectrophotometry, chromatography, and electrochemical techniques. The collected data is then analyzed using mathematical models and statistical methods to extract meaningful information, such as reaction rates, equilibrium constants, and activation energies.
Time and Concentration Data Collection in Chemical Reactions
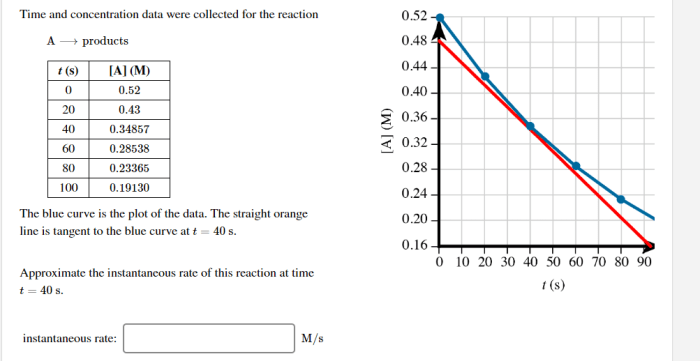
The collection of time and concentration data is crucial for understanding the kinetics and mechanisms of chemical reactions. Various methods are employed to obtain this data, enabling researchers to analyze reaction rates, determine equilibrium concentrations, and investigate the factors influencing these parameters.
Time and Concentration Data Collection Methods
Spectrophotometry:This technique utilizes the absorption or emission of light by chemical species to determine their concentrations. By measuring the absorbance or fluorescence intensity at specific wavelengths, researchers can quantify the presence and concentration of reactants and products.
Chromatography:Chromatographic techniques, such as high-performance liquid chromatography (HPLC) and gas chromatography (GC), separate and analyze mixtures of compounds based on their interactions with a stationary phase. This allows for the identification and quantification of individual components in a reaction mixture.
Data Analysis and Interpretation
The collected time and concentration data are analyzed using mathematical models and statistical methods to extract meaningful information. Common approaches include:
- Rate laws:These equations describe the relationship between the reaction rate and the concentrations of reactants.
- Equilibrium constants:These values represent the ratio of product concentrations to reactant concentrations at equilibrium.
- Regression analysis:This statistical technique helps determine the best-fit model that describes the experimental data.
Factors Affecting Time and Concentration, Time and concentration data were collected for the reaction
Several factors can influence the time and concentration of a chemical reaction, including:
- Temperature:Higher temperatures generally increase reaction rates.
- pH:The acidity or basicity of the reaction medium can affect the ionization of reactants and products, influencing their reactivity.
- Catalyst concentration:Catalysts are substances that increase reaction rates without being consumed. Increasing catalyst concentration typically leads to faster reactions.
Applications and Implications
Time and concentration data have numerous practical applications in various fields, such as:
- Optimizing reaction conditions:This data allows researchers to identify the optimal conditions (e.g., temperature, pH, catalyst concentration) for specific reactions.
- Designing experiments:By understanding the factors affecting reaction time and concentration, scientists can design experiments to achieve desired outcomes.
- Predicting reaction outcomes:Kinetic models based on time and concentration data can predict the concentrations of reactants and products at different time points.
FAQ Guide: Time And Concentration Data Were Collected For The Reaction
What is the importance of time and concentration data in chemical reactions?
Time and concentration data provide insights into the reaction rates, equilibrium constants, and activation energies, which are essential for understanding and predicting the behavior of chemical reactions.
How are time and concentration data collected?
Time and concentration data are collected using various methods, such as spectrophotometry, chromatography, and electrochemical techniques, which allow researchers to monitor the changes in concentration over time.
How is time and concentration data analyzed?
Time and concentration data are analyzed using mathematical models and statistical methods to extract meaningful information, such as reaction rates, equilibrium constants, and activation energies.